Celebrating the National Consciousness Week Against Counterfeit Medicines (NCWACM) 2022, the Faculty of Pharmacy, as a part of the country’s Food and Drug Administration, held an academic symposium to raise awareness and to combat the distribution of counterfeit medicines on November 15, 2022 at the Central Laboratory Auditorium.
Faculty of Pharmacy Dean Prof. Aleth Therese L. Dacanay, PhD reminded the Thomasian pharmacists of their vital role to play in this campaign as they promote vigilance and disseminate proper information to the public regarding the harmful effects of counterfeit medicines. “With the many counterfeit medicines that disguise themselves as the real thing, packaged beautifully and sold in stores […] we have a great responsibility to make the public more aware of the proliferation of the counterfeit medicines due to the health risks that accompany them,” Dean Dacanay said in her opening message.
Legal Framework against Counterfeit Drugs
Referring the production of counterfeit drugs as an organized crime that happens worldwide, Atty. Langley P. Gratuito of the Legal Services Support Center of Food and Drug Administration talked about the Republic Act 8203, or the Special Law on counterfeit Drugs, which took effect on October 26, 1996. FDA was the government agency that spearheaded its provisions.
Atty. Gratuito warned the public that counterfeit drugs could be bought in online shopping platforms, noting the gap in the legislation for there has not been any law established yet to combat online transactions.
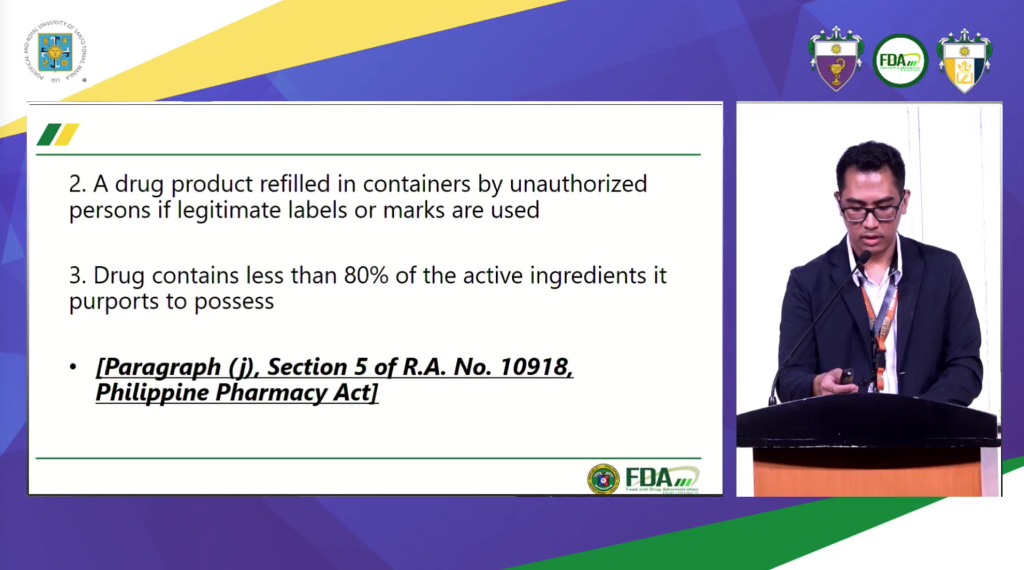
According to the said law, drugs, on one hand, is defined as any chemical compound or biological substance, other than food, for us in the treatment, prevention or diagnosis of disease in man or animals. On the other hand, counterfeit drugs is defined as medicinal products with the correct ingredients but not in the amounts provided hereunder; contains wrong ingredients; without active ingredients; and insufficient quantity of active ingredient which results in the reduction of the drug’s safety efficacy, quality, strength or purity. It is a drug which is deliberately and fraudulently mislabed with respect to identity and/or source or fake packaging. Moreover, its is also considered counterfeit when the drugs’s labelling and container is without authorization trademarks, tradename or other identification mark or imprint or any likeness owned of registered in the Intellectual Property Office.
Atty. Gratuito likewise laid down the liabilities for violating the said law:
1) Permanent closure of establishment concerned and revocation of its business license;
2) A fine of not less than P100, 000 but not more than P500,000;
3) Upon the order of the Supreme Court, forfeiture, confiscation, and destruction of products found to be counterfeit and the equipment, instruments, and other articles used in violation of this law;
4) Filing of an appropriate proceedings against the registered pharmacist with the Professional Regulations Commission for cancellation of the professional license;
5) Filing of criminal charges against the violators, which can be instituted independently from the administrative case; and
6) Permanent disqualification of the person concerned, whether natural or juridicial, from owning or operating an established engaged in any business activity under the supervision of the Bureau.
In terms of the criminal penalties:
1) Imprisonment of not less than six months and one day but not moe than six years for mere possession of counterfeit drugs;
2) imprisonment of six years and one day but not more than 10 years or a fine not less than P100, 000 but not more than P500, 000 or both such imprisonment and fine at the discretion of the court in any other case in the Section 4 of the law;
3) Imprisonment of not less than six months and one day but not more than two years and four months if the counterfeit drug is intended for animals;
4) Imprisonment of not less than six years and one day, but not more than 10 years for any manufacturer, seller or distributor who shall conceal, substitute, dispose or destroy any drug as may have been segregated and sealed by the Bureau, or who shall break, alter or tamper any mark or seal used by the Bureau to identify those segregated drugs as provided for under Section 6 of the law;
5) If, as a result of the use of the drug found to be counterfeit, the illness sought to be cured is aggravated or physical injury or suffering results therefrom, a punishment of imprisonment from 12 years to fifteen years and a fine ranging from P100, 000 to P500, 000 shall be meted out;
6) Should a counterfeit drug be the proximate cause of a death of a victim, who unknowingly purchased and took a counterfeit drug, the penalty of life imprisonment and a fine of P500, 000 to P5 million shall be imposed.
War on counterfeit drugs
Anna Macion-Tecson, Special Investigator of Field Regulatory Enforcement Unit, lectured on the fight against counterfeit medicine. She noted that before they conduct a buy-bust operation, they coordinate first with the law enforcement agencies like Philippine National Police. “This is for the safe and successful implementation of any enforcement activities, so dito rin po namin maa-identify kung ano po yung role ni FDA kung kami po ba ang magiging complainant dito, or seizure officer […] but mostly, PNP is the one who arrests the violator of RA 8203 and FDA is the one that initiates the enforcement activities,” Tecson said.
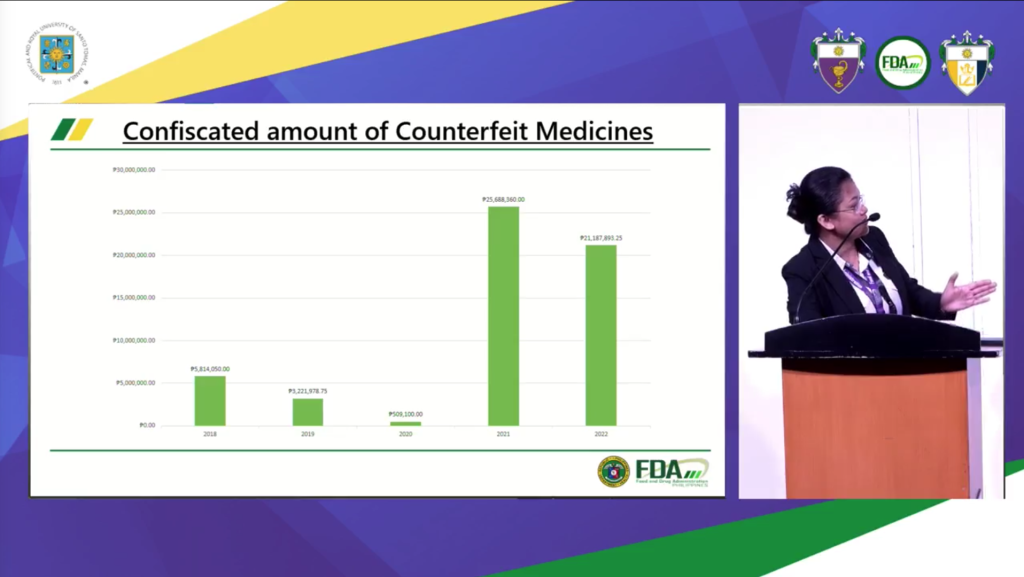
In her presentation, P25,688,360 worth of counterfeit drugs were seized in 2021 and P21,187,893.25 worth of said illegal items were confiscated during their buy-bust operations in 2022.
Mark Anthony A. Polpol, of NCR – Regional Field Office, lectured on the local situation on counterfeit drugs. For Polpol, FDA sends out advisories on partner groups and Local Government Units to help their relentless campaign on counterfeit drugs. “We inform the public through FDA advisories about unregistered or counterfeit. And also, through these advisories, FDA encourages the LGUs and law enforcement agencies to ensure that these products are not sold or made available in their localities or their area of jurisdiction,” Pulpol said.
Meanwhile, sharing how the public can protect themselves in acquiring the said items, FDA Regulation Officer Sheralyn A. Opeña, RPH laid down a few guides in purchasing genuine drug products, namely 1) Visual Inspection, 2) Source, 3) Price, 4) Unexpected side-effects.
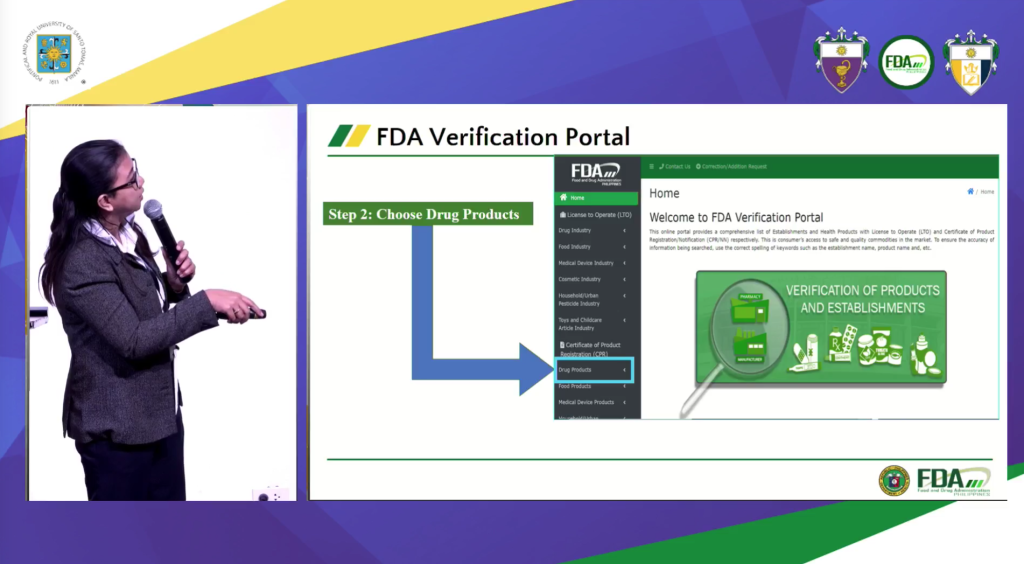
She also shared the FDA Verification Portal of www.fda.gov.ph to check if the product is registered. “Puwede niyong i-check kung may license to operate yung establishment or registered ang drug product,” Opeña said.
The University, being one one with the Food and Drug Administration in this joint advocacy, hosted this event to arm the general public with the right information and professional advices from the experts panel, who belong to the regulating body.




